Chance based strategy in VMP. This is certainly also described in cleaning validation report distinct to the cleaning method, the gear along with the merchandise. Normally, shorter frequencies originally of regime cleaning (schedule production) are sensible and elongen the frequency information based.
It’s also a necessity that the validation process doesn't assist the growth of microbes. In analyzing When the validation method has supported microbial progress, the storage on the products right before cleaning and right after cleaning is frequently thought of to make a decision whether or not they support microbial development.
The ECA offers a variety of cost-free GMP newsletters for which you'll subscribe to according to your needs.
a) Location tough to clear and which are fairly clean could be evaluated by immediate floor sampling method (Swab Method), bringing about developing a level of contamination or residue for each supplied region i.e. 60 – a hundred in2. The residue that is certainly dried out or is insoluble might be sampled by Swab Method.
Equipment cleaning validation may very well be carried out concurrently with true output actions all through procedure development and bulk producing. Validation programs needs to be ongoing as a result of total-scale commercial creation
A trusted cleaning validation application is essential to GMP producing and assists empower a production unit to deliver quality solutions on time and in total to market place.
Swab sampling won't address your entire devices area location hence web sites should be chosen with care. It is important that, for a minimum amount, the swab sites represents worst situation locations on the machines and that The end result is then extrapolated to account for the total solution Call surface region.
helo ankur you should tell me for just a devices cosecutive three batches of very same item is necessary or not
For most circumstances, the choice of the limit check here is predicated on affected person security; on the other hand, you will discover other components that can affect the selection, demanding additional evaluation. The technical and high quality individuals are to blame for the ultimate decision with appropriate justification.
The acceptance standards, including the rationale for environment the precise limits; Other solutions, processes, and equipment for which the planned validation is legitimate in accordance to the “bracketing” strategy; and
• periodic analysis and revalidation of the amount of batches manufactured among cleaning validations.
The Validation of the Cleaning Procedures is creating documented evidence which the method is effective and able for removing the contaminants connected to preceding products and solutions, residues of cleaning agents as well as the control of opportunity microbial contaminants.
Once the acceptance of any click here transform in accordance with the method, it is necessary to revalidate the Cleaning Technique.
The people today conducting the process needs to be properly trained prior to they start the whole process of cleaning method validation. They needs to have understanding of cleaning process, normal running process and validation protocol.
 Ben Savage Then & Now!
Ben Savage Then & Now! Tatyana Ali Then & Now!
Tatyana Ali Then & Now! Mike Vitar Then & Now!
Mike Vitar Then & Now!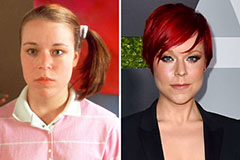 Tina Majorino Then & Now!
Tina Majorino Then & Now! Mary Beth McDonough Then & Now!
Mary Beth McDonough Then & Now!